Fourth Lab Report: JAGUNG PANDAN
Title: Soil Sieve and Nutrient Analysis
Date of Submission: 28th April 2018
Lecturer: DR. DIANA DEMIYAH MOHD HAMDAN
Date of Submission: 28th April 2018
Lecturer: DR. DIANA DEMIYAH MOHD HAMDAN
NAME
|
MATRIC NUMBER
|
PAVITRA A/P MURUGAYAH
|
BS17160700
|
NURUL NATASYAH BINTI KANAPIA@HANAFIAH
|
BS17110546
|
KONG WAN LING
|
BS17110429
|
NURFATIN SOFEA BINTI MOHD HELMI
|
BS17110574
|
SOW XIAO HUI
|
BS17110464
|
AARON CHIN VUI CHANG
|
BS17160670
|
Soil Sieve Analysis
1.0 Introduction
Sieve
analysis is an analytical technique used to determine the particle size
distribution of the coarse and fine aggregates. The technique involves the
layering of sieves with different grades of sieve opening sizes. The size
distribution is critical crucial to the way the material performs in use and
sieve analysis can be performed on any type of non-organic or organic granular
materials including sands, crushed rock, clays, granite, feldspars, coal, soil,
a wide range pf manufactured powders, grain and seeds then down minimum size
depending on exact method. The amount is determined by largest size of
aggregate is placed upon the top of a sieves and shaken by mechanical means for
a period of the time. The top sieve has the largest screen openings and the screen
opening sizes decreases with each sieve down to the bottom sieve which has
smallest opening size screen for the type of material specified. Sieve analysis
is important for analyzing materials because particle size distribution can
affect a wide range of properties such as the strength of concrete, the
solubility of a mixture, surface area properties and even their taste.
2.0 Objectives
To
determines the relative proportions of different grain sizes as they are
distributed among certain size ranges.
3.0 APPARATUS AND MATERIALS
Air-dried
soils
Stack
of sieves including pan and cover
Weighing
balance
Mechanical
sieve shaker
Brush
Pestle
and mortar
Tray
4.0 PROCEDURE
- Tree
roots, pieces of bark and rocks were removed from the soil samples.
- Clumps
of air-dried soils was break by hand or pestle and mortar were used before
air-dried soils sample were sieve.
- The
total weight of the soils sample were measured before sieve.
- Five
size of mesh sieve (one of the sieve=63µm mesh size) were selected.
- The
sieves were ensure be cleaned.The brush was use to poke the soil particles
that stuck in the openings without injuring the mesh.
- A
stack of sieves were prepared on the mechanical sieve shaker.The order on
the larger opening size until smaller opening size of sieves were ensure
in the correct position.The pan were set first in the stack and the top of
the biggest mesh size sieve was covered by cover.
- The
soil sample was pour and the cover was place on it.
- The
clamps were fixed.
- A
tray was placed below the opening of the pan to collect the finest
particle.
- The
time was adjusted to 15 minutes and the shaker was get going on 40-50.
- After
the shaker had stopped,the mass of each sieve and retained soil were mass.
- The
brush was used to poke out the particles that stuck on the mesh (if any)
and it was collected.
- The
soil collected were labelled and weight on their mass.
- The
results were recorded and calculated.
5.0 Result
and Discussion
Mangrove Soil
Total Mass= 100g
Total Mass= 100g
Table 5.1: Sieve Analysis Result of Mangrove Soil
No. Sieve
|
Sieve Opening Mesh
Size (mm)
|
Mass of soil retained
on each sieve (g)
|
Percentage of mass
retained on each sieve, Rn (%)
|
Cumulative percent
retained (% Cumulative Passing= 100% - % Cumulative Retained) (%)
|
Percentage of Finer
(100 - ∑Rn)
(%)
|
| 10 |
2.000
|
3.806
|
3.806
|
3.806
|
96.194
|
| 18 |
1.000
|
1.851
|
1.851
|
5.657
|
94.343
|
| 20 |
0.600
|
3.724
|
3.724
|
9.381
|
90.619
|
| 35 |
0.500
|
1.891
|
1.891
|
11.2712
|
88.728
|
| 230 |
0.063
|
69.024
|
69.024
|
80.295
|
19.705
|
Pan
|
- |
19.705
|
19.705
|
100.000
|
0.000
|
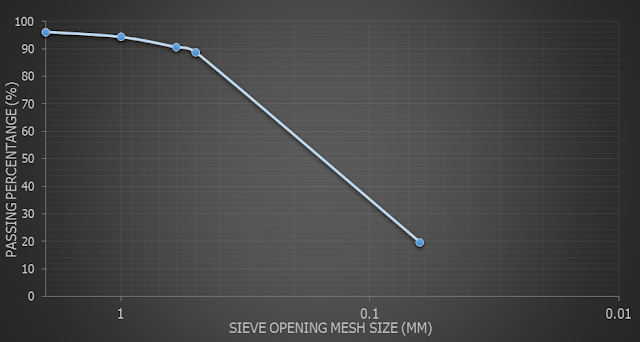
Figure 5.1: Graph of Passing Percentage against Sieve Size for Mangrove
Soil
Lake of Residential College E Soil
Total Mass= 100g
Total Mass= 100g
Table 5.2: Sieve Analysis Result of Lake of Residential College E Soil
No. Sieve
|
Sieve Opening Mesh
Size (mm)
|
Mass of soil retained
on each sieve (g)
|
Percentage of mass
retained on each sieve, Rn (%)
|
Cumulative percent
retained (% Cumulative Passing= 100% - % Cumulative Retained) (%)
|
Percentage of Finer
(100 - ∑Rn)
(%)
|
| 10 |
2.000
|
3.200
|
3.200
|
3.200
|
96.801
|
| 18 |
1.000
|
2.600
|
2.600
|
5.800
|
94.201
|
| 20 |
0.600
|
2.440
|
2.440
|
8.240
|
91.761
|
| 35 |
0.500
|
1.657
|
1.657
|
9.896
|
90.104
|
| 230 |
0.063
|
66.625
|
66.625
|
76.522
|
23.479
|
Pan
|
- |
23.479
|
23.479
|
100.000
|
0.000
|
Figure 5.2: Graph of Passing Percentage against Sieve Size for Lake of
Residential College E Soil
FSSA Soil
Total Mass= 100g
Total Mass= 100g
Table 5.3: Sieve Analysis Result of FSSA Soil
No. Sieve
|
Sieve Opening Mesh
Size (mm)
|
Mass of soil retained
on each sieve (g)
|
Percentage of mass
retained on each sieve, Rn (%)
|
Cumulative percent
retained (% Cumulative Passing= 100% - % Cumulative Retained) (%)
|
Percentage of Finer
(100 - ∑Rn)
(%)
|
| 10 |
2.000
|
19.006
|
19.006
|
19.006
|
80.994
|
| 18 |
1.000
|
17.118
|
17.118
|
36.124
|
63.876
|
| 20 |
0.600
|
10.799
|
10.799
|
46.923
|
53.077
|
| 35 |
0.500
|
3.953
|
3.953
|
50.876
|
49.124
|
| 230 |
0.063
|
34.937
|
34.9371
|
85.813
|
14.187
|
Pan
|
- |
14.187
|
14.187
|
100.000
|
0.000
|
Figure 5.3: Graph of Passing Percentage against Sieve Size for FSSA Soil
Mountain Soil
Total Mass= 100g
Total Mass= 100g
Table 5.4: Sieve Analysis Result of Mountain Soil
No. Sieve
|
Sieve Opening Mesh
Size (mm)
|
Mass of soil retained
on each sieve (g)
|
Percentage of mass
retained on each sieve, Rn (%)
|
Cumulative percent
retained (% Cumulative Passing= 100% - % Cumulative Retained) (%)
|
Percentage of Finer
(100 - ∑Rn)
(%)
|
| 10 |
2.000
|
17.276
|
17.276
|
17.276
|
82.724
|
| 18 |
1.000
|
13.728
|
13.728
|
31.004
|
68.996
|
| 20 |
0.600
|
8.308
|
8.308
|
39.312
|
60.688
|
| 35 |
0.500
|
3.680
|
3.680
|
42.992
|
57.008
|
| 230 |
0.063
|
40.756
|
40.756
|
83.748
|
16.252
|
Pan
|
- |
16.252
|
16.252
|
100.000
|
0.000
|
Figure 5.4: Graph of Passing Percentage against Sieve Size for Mountain
Soil
Sandy Soil
Total Mass= 100g
Total Mass= 100g
Table 5.5: Sieve Analysis Result of Sandy Soil
No. Sieve
|
Sieve Opening Mesh
Size (mm)
|
Mass of soil retained
on each sieve (g)
|
Percentage of mass
retained on each sieve, Rn (%)
|
Cumulative percent
retained (% Cumulative Passing= 100% - % Cumulative Retained) (%)
|
Percentage of Finer
(100 - ∑Rn)
(%)
|
| 10 |
2.000
|
1.001
|
1.001
|
1.001
|
98.999
|
| 18 |
1.000
|
1.140
|
1.140
|
2.140
|
97.860
|
| 20 |
0.600
|
1.336
|
1.336
|
3.477
|
96.524
|
| 35 |
0.500
|
1.278
|
1.278
|
4.755
|
95.246
|
| 230 |
0.063
|
2.989
|
2.989
|
7.743
|
92.257
|
Pan
|
-
|
92.257
|
92.257
|
100.000
|
0.000
|
Figure 5.5: Graph of Passing Percentage against Sieve Size for Sandy
Soil
6.0 Discussion
Sieve analysis test helps to
determine the particle size distribution of the coarse and fine aggregates. The
calculated by the combination of several separate elements of the rocks is
known as aggregates. Meanwhile, a sieve analysis can be performed on any either
non-organic or organic granular materials including sands, crushed rock, clays,
granite, feldspars, coal, soil, a wide range of manufactured powders, grain and
seeds, down to a minimum size depending on the exact method. In this
experiment, there are five types of dry soils that will be used for the sieve
analysis which are Mangrove soil, FSSA soil, Lake of Residental College E,
Sandy soil and Mountain soil. According to British Soil Classification System, the
soils are classified according to different sizes which are further divided
into course, medium and fine sub-groups.
From the graph above, it
shows that Lake of Residental College E have almost same pattern as Mangrove
soil while FSSA soil have almost same pattern as Mountain soil. Each of the soils
have different type of percent passing through 0.063 mm. It is clearly that the
finest soil of percentage is sandy soil with percentage of 92.257. Only a small
percentage with 14.187 % of FSSA soil that can pass through the finest mesh
sieve.
From the previous
results, FSSA soil contains 0.20 cm of sand (3.08%) and 6.30 cm of silt (96.9%)
and 0% of clay from the soil jar test. According to the soil texture triangle,
it can be said that the FSSA soil is a silt soil. According to the theory,
silt particles measure between 0.05 and 0.002 mm, suppose be the finest
one. However, the type of FSSA soil that was determined through sieve analysis
cannot be supported by the results of soil texture analysis and jar test
analysis where both test have shown that the FSSA soil is a silt soil
whereas the sieve analysis show it was the least soil in the percentage of
finer.
This is because it can be
shown that the soil with the highest percentage finer would be soil samples
from silty clay loam. However, through the sieve analysis test sandy soil
recorded the finest soil in the finest sieve opening mesh size as the
percentage of finer soils that passed through the 63 microns sieve mesh was the
highest (92.257%), followed by Lake of Residental College E soil (23.479%),
mangrove soil (19.705%) and mountain soil (16.252%). This can be said that, as
the sizes of the mesh sieves decreases, only finer particles such as clay and
silt which the size ranges are smaller than sand would be able to pass through
the sieves. So, it can be said that our sandy soil may the finest seamless sand
which is the finest among the all types of soil (Haseeb Jamal, 2005).
Thus, method of using
sieve analysis to calculate the mass and percentage of retained soil sometimes may
not really accurate because there will be some particles that stuck in the
opening of sieves and hardly to be removed as well as some of the soil
particles may finer that the sieve opening mesh size. Furthermore, the results
can also be affected by spilling of some particles contents during the
separating of tightly fit the sieves from the nest after shaking. Therefore, to
prevent and ensure the minimal loss of soil during the experiment, the
difference between combined mass of all retained soil and pre-sieve soil can be
compared. The larger the differences, the more soil is loss during the
experiment which indicates inaccuracy in the result.
7.0 Conclusion
In conclusion, sandy soil recorded as
the highest passing percentage (92.257%) which means the most the finest size
particles pass through the sieve analysis. Then followed by lake of Residential
College E soil (23.479%), mangrove soil (19.705%) and mountain soil (16.252%).
For FSSA soil, recorded as the lowest passing percentage among these five soils
which is 14.187%.
8.0 Reference
A. Blaud, M. Menon et al.
(2016). Effects of Dry and Wet Sieving of Soil on Identification
and Interpretation of Microbial Community Composition. Retrived on 29
April 2018 from https://www.sciencedirect.com/science/article/pii/S0065211316301122#.
Antonio Girona-García,
Oriol Ortiz-Perpiñá etal. (2017). Effects of prescribed burning on soil
organic C, aggregate stability and water repellency in a subalpine shrubland:
Variations among sieve fractions and depths. Retrieved on 28.04.2018
from https://www.sciencedirect.com/science/article/pii/S0341816218301000.
Haseeb
Jamal. Sieve Analysis & Particle Size
Analysis. Retrieved on 28.04.2018 from https://www.aboutcivil.org/Sieve-analysis-and-soil-classification.html.
Leslie
Davidson, Sarah Springman. (2000). Soil
Description and Classification. Retrieved on 29.04.2018 from http://environment.uwe.ac.uk/geocal/SoilMech/classification/default.htm.
Soil Nutrient Analysis
1.0 Introduction
Plant
growth and development largely depend on the combination and concentration of
mineral nutrients available in soil. Plants often face significant challenges
in obtaining an adequate supply of these nutrients to meet the demands of basic
cellular processes due to their relatively immobility. A deficiency of any one
of them may result in decreased plant productivity and fertility. Symptoms of
nutrients deficiency may include stunted growth, death of plants tissue or
yellowing of the leaves caused by reduced by a reduced production of chlorophyll,
a pigment needed for photosynthesis.
Nutrient deficiency can have a
significant impact on agriculture, resulting in reduced crop yield or reduced
plant quality. Nutrients deficiency can also lead to reduced overall
biodiversity since plants serve as the producers that support most food webs.
Changes in the climate and atmosphere
can have serious effects on plants, including changes in the availability of
certain nutrients. In a world of continual global climate change, it is very
important to understand the strategies that plants have evolved to allow them
to cope with some of these obstacles.
Two classes of nutrients are
considered essential for plant which are macronutrients and micronutrients. Macronutrients
are building blocks of crucial cellular components like proteins and nucleic. They
required in large quantities. Nitrogen, phosphorus, magnesium and potassium are
some of the most important macronutrients.
Micronutrients including iron, zinc, manganese
and copper are required in very small amount. Micronutrients also often
required as cofactor for enzymes. Mineral nutrients are usually obtained from
the soil through plants roots, but many factors can affect the efficiency of
nutrient acquisition. The chemistry and composition of certain soils can make
it harder for the plants to absorb nutrients. The nutrients may not be
available in certain soils, or may be present in forms that the plants cannot
use. Soil properties like water content, pH and compaction may exacerbate these
problems.
Plants are known to show different
responses to different specific nutrients deficiencies and the responses can
vary between species.
2.0 Objective
1)
To
identify the macronutrients in plants such nitrogen (N), phosphorus (P) and
sulphate (S).
3.0 Apparatus
and Materials
1. 20 gram
dried soil samples
2. Distilled water
3. 200 ml glass beaker
4. 0.45µm membrane filter paper
5. Vacuum pump
6. Magnetic field
7. Stirrer plate
8. Spatula
9. High density polyethylene (HDPE) bottle
10. Machine HACH
11. Powder pillow for Code (680 Sulphate). (490 P React. PV- Phosphorus) and (355N, Nitrate HR PP)
2. Distilled water
3. 200 ml glass beaker
4. 0.45µm membrane filter paper
5. Vacuum pump
6. Magnetic field
7. Stirrer plate
8. Spatula
9. High density polyethylene (HDPE) bottle
10. Machine HACH
11. Powder pillow for Code (680 Sulphate). (490 P React. PV- Phosphorus) and (355N, Nitrate HR PP)
4.0 Procedures
1. Avoid
handling the soil with your bare hands and the gloves is wear while handling.
2. The soil that has been air-dried outside of the lab is weighed. The soil is touched to ensure complete dried out of moisture.
3. 20g of dried soil samples is taken and put in beaker separately for each type of soil.
4. 50ml of distilled water is added together with the soil samples.
5. The magnetic stirrer is using to mix the sample solution well for 20 minutes.
6. The mixture is allowed to stand undisturbed for at least 10 minutes to allow the fine particles in the soil to settle out. The clarity of the solution will vary, the clear the better,
7. Then, the solution is filtered using the 0.45µm membrane filter paper vacuum. Filter solution which is clear liquid first compare to murky ones. The HDPE bottles is stored for macronutrient analysis using the HACH kit.
8. Next, three nutrient are analysed using the HACH kit.
9. Wearing the gloves all the time when conducting experiment by using HACH kit.
10. The nutrient is analysed with code (680 Sulphate), (490 P React. PV-Phosphorus) and (355N, Nitrate HR PP).
11. The readings for each nutrients is taken and averaged.
12. After solution had been analysed, the solution is not discard in the sink. Collect in a beaker first before discard into a proper waste storage.
2. The soil that has been air-dried outside of the lab is weighed. The soil is touched to ensure complete dried out of moisture.
3. 20g of dried soil samples is taken and put in beaker separately for each type of soil.
4. 50ml of distilled water is added together with the soil samples.
5. The magnetic stirrer is using to mix the sample solution well for 20 minutes.
6. The mixture is allowed to stand undisturbed for at least 10 minutes to allow the fine particles in the soil to settle out. The clarity of the solution will vary, the clear the better,
7. Then, the solution is filtered using the 0.45µm membrane filter paper vacuum. Filter solution which is clear liquid first compare to murky ones. The HDPE bottles is stored for macronutrient analysis using the HACH kit.
8. Next, three nutrient are analysed using the HACH kit.
9. Wearing the gloves all the time when conducting experiment by using HACH kit.
10. The nutrient is analysed with code (680 Sulphate), (490 P React. PV-Phosphorus) and (355N, Nitrate HR PP).
11. The readings for each nutrients is taken and averaged.
12. After solution had been analysed, the solution is not discard in the sink. Collect in a beaker first before discard into a proper waste storage.
4.1 Procedure
for nutrient analysis code (680 Sulphate)
1. The stored
programs is pressed.
2. Code (680 Sulphate) is selected for the test.
3. A square sample cell is filled with 10ml of sample.
4. To prepare the samples, the contents of one SulfaVer 4 Reagent Powder Pillow is added to the sample cell. Then, swirled vigorously to dissolve powder and white turbidity will form if sulphate is present.
5. The timer is press and a five minute reaction period will begin. Do not disturb the cell during this time.
6. A second square sample cell is filled with 10ml of sample.
7. When the timer expires, the blank is inserted into the cell holder with the line facing right. Next, press Zero and the display will showed.
8. Within five minutes after the timer expires, the prepared sample is inserted into the cell holder with the filled liner facing right. Press read and the results is recorded. Next, the sample cells is cleaned with a soap and brush.
2. Code (680 Sulphate) is selected for the test.
3. A square sample cell is filled with 10ml of sample.
4. To prepare the samples, the contents of one SulfaVer 4 Reagent Powder Pillow is added to the sample cell. Then, swirled vigorously to dissolve powder and white turbidity will form if sulphate is present.
5. The timer is press and a five minute reaction period will begin. Do not disturb the cell during this time.
6. A second square sample cell is filled with 10ml of sample.
7. When the timer expires, the blank is inserted into the cell holder with the line facing right. Next, press Zero and the display will showed.
8. Within five minutes after the timer expires, the prepared sample is inserted into the cell holder with the filled liner facing right. Press read and the results is recorded. Next, the sample cells is cleaned with a soap and brush.
4.2 Procedure
for nutrient analysis code (490 P React. PV-Phosphorus)
1. The stored
programs is pressed.
2. Code (490 P React. PV-Phosphorus) is selected for the test.
3. A square sample cell is filled with 10ml of sample.
4. To prepared the samples, the contents of one PhosVer 3 phosphate Powder Pillow is added to the cell. Immediately stopper and shake vigorously for 30 minutes.
5. The timer is press and a two minutes reaction period will begin. If the samples was digested using the Acid Persulphate digestion, a ten minute reaction period is required.
6. A second square sample cell is filled with 10ml of sample.
7. When the timer expires, the blank is wiped and inserted it into the cell holder with the filled line facing right, press Zero and the display will showed.
8. The prepared sample is wiped and inserted it into the cell holder with the filled line facing right. Press read and the results is recorded.
2. Code (490 P React. PV-Phosphorus) is selected for the test.
3. A square sample cell is filled with 10ml of sample.
4. To prepared the samples, the contents of one PhosVer 3 phosphate Powder Pillow is added to the cell. Immediately stopper and shake vigorously for 30 minutes.
5. The timer is press and a two minutes reaction period will begin. If the samples was digested using the Acid Persulphate digestion, a ten minute reaction period is required.
6. A second square sample cell is filled with 10ml of sample.
7. When the timer expires, the blank is wiped and inserted it into the cell holder with the filled line facing right, press Zero and the display will showed.
8. The prepared sample is wiped and inserted it into the cell holder with the filled line facing right. Press read and the results is recorded.
4.3 Procedure
for nutrient analysis code (355N, Nitrate HR PP)
1. The stored
programs is pressed.
2. Code (355N, Nitrate HR PP) is selected for the test.
3. A square sample cell is filled with 10ml of sample.
4. To prepare samples, the contents of one NitraVer 5-Nitrate Reagent Powder Pillow is added.
5. The timer is press and a one minute reaction period will begin.
6. The cell is shook vigorously until the timer expires.
7. When the timer expires, press timer again and a five minute reaction will begin. An amber colour will develop if nitrate is present.
8. Next, when the timer expires, a second square samples cell is filled with 10ml of samples.
9. The blank is wiped and inserted it into the cell holder with the fill line facing right.
10. Then, press Zero and the display will showed,
11. Within one minute after the timer expires, the prepared samples is wiped and inserted it into the cell holder with the filled line facing right. Press read and results is recorded.
2. Code (355N, Nitrate HR PP) is selected for the test.
3. A square sample cell is filled with 10ml of sample.
4. To prepare samples, the contents of one NitraVer 5-Nitrate Reagent Powder Pillow is added.
5. The timer is press and a one minute reaction period will begin.
6. The cell is shook vigorously until the timer expires.
7. When the timer expires, press timer again and a five minute reaction will begin. An amber colour will develop if nitrate is present.
8. Next, when the timer expires, a second square samples cell is filled with 10ml of samples.
9. The blank is wiped and inserted it into the cell holder with the fill line facing right.
10. Then, press Zero and the display will showed,
11. Within one minute after the timer expires, the prepared samples is wiped and inserted it into the cell holder with the filled line facing right. Press read and results is recorded.
5.0 Result
Table 5.1: Result of Nutrient
Analysis for Each Soil Sample
TYPE OF SOIL
|
FIRST READING
|
SECOND READING
|
THIRD READING
|
AVERAGE
|
|
Mangrove
|
680 Sulphate
|
>3.50!
|
>3.50!
|
>3.50!
|
>3.50!
|
355N, Nitrate HR PP
|
3.60
|
3.60
|
3.60
|
3.60
|
|
490 P React. PV- Phosphorus
|
0.38
|
0.38
|
0.38
|
0.38
|
|
Mountain
|
680 Sulphate
|
1.00
|
1.00
|
1.00
|
1.00
|
355N, Nitrate HR PP
|
2.80
|
2.80
|
2.80
|
2.80
|
|
490 P React. PV- Phosphorus
|
0.19
|
0.19
|
0.19
|
0.19
|
|
Lake of Residential College E
|
680 Sulphate
|
33.00
|
33.00
|
33.00
|
33.00
|
355N, Nitrate HR PP
|
Over detection limit
|
Over detection limit
|
Over detection limit
|
Over detection limit
|
|
490 P React. PV- Phosphorus
|
0.51
|
0.51
|
0.51
|
0.51
|
|
FSSA
|
680 Sulphate
|
18.00
|
18.00
|
18.00
|
18.00
|
355N, Nitrate HR PP
|
3.90
|
3.90
|
4.00
|
3.93
|
|
490 P React. PV- Phosphorus
|
0.88
|
0.91
|
0.92
|
0.90
|
|
Sandy
|
680 Sulphate
|
11.00
|
11.00
|
11.00
|
11.00
|
355N, Nitrate HR PP
|
4.10
|
4.10
|
4.10
|
4.10
|
|
490 P React. PV- Phosphorus
|
0.28
|
0.30
|
0.32
|
0.30
|
6.0 Discussion
Soil is a
major source of nutrients needed by plants for growth. Plants need at least 17
nutrients. These include the macronutrients nitrogen (N), phosphorus (P),
potassium (K), calcium (Ca), magnesium (Mg) and sulphur (S) and the
micronutrients chlorine (Cl), boron (B), iron (Fe), manganese (Mn), copper
(Cu), zinc (Zn), nickel (Ni) and molybdenum (Mo). These are generally obtained
from the soil. Crop production is often limited by low phytoavailability of
essential mineral elements or the presence of excessive concentrations of
potentially toxic mineral elements. The macronutrient are needed in large
amount whereas the micronutrient are needed in small amount.
Nitrogen
is a key element in plant growth. It is found in all plant cells, in plant
proteins and hormones, and in chlorophyll. Atmospheric nitrogen is a source of
soil nitrogen. Some plants such as legumes fix atmospheric nitrogen in their
roots; otherwise fertiliser factories use nitrogen from the air to make
ammonium sulphate, ammonium nitrate and urea. When applied to soil, nitrogen is
converted to mineral form, nitrate, so that plants can take it up. Soils high
in organic matter such as chocolate soils are generally higher in nitrogen than
podzolic soils. Nitrate is easily leached out of soil by heavy rain, resulting
in soil acidification.
Phosphorus
(P) is important in cell division, root development, flowering and fruiting.
Potassium (K) increases vigour and disease resistance of plants, helps form and
move starches, sugars and oils in plants, and can improve fruit quality. On the
other hand, sulphur is a structural component of some amino acids (including
cysteine and methionine) and vitamins, and is essential for chloroplast growth
and function; it is found in the iron-sulphur complexes of the electron
transport chains in photosynthesis. It is needed for N2 fixation by legumes,
and the conversion of nitrate into amino acids and then into protein. It is
also responsible for many flavours and odours compounds in plants such as the
aroma of onions and cabbage.
To
determine the nutrient content, 680 Sulphate reagent was used to determine the
amount of sulphate (sulphur) content in the soil , 490 P React PV-phosphorus
was used to determine the amount of phosphorus content and 355 N, Nitrate HRPP
was used to determine the nitrate (nitrogen) content. Firstly, the mangrove
soil sulphate content is >3.5!, the nitrate content is 3.6 and the
phosphorus content is 0.38 whereas for the mountain soil, the sulphate content
is 1, the nitrate content is 2.8 and the phosphorus content is 0.19. For the
soil from the lake of Residential College E , it has the highest amount of
sulphate content compared to the rest which is 33, the nitrate content is over
the detection limit which is also known as over the detection range and the
phosphorus content is 0.51. Next, FSSA soil has got the most phosphorus content
in its soil which is 0.90, the sulphate content is 18 and the nitrate content
is 3.93. Lastly, the sandy soil has the highest content of nitrate which is
4.1, the sulphate content is 11 and the phosphorus content is 0.3.
Nitrogen
deficiency most often results in stunted growth, slow growth, and chlorosis.
Nitrogen deficient plants will also exhibit a purple appearance on the stems,
petioles and underside of leaves from an accumulation of anthocyanin pigments.
Besides that, phosphorus deficiency in plants is characterized by an intense
green coloration or reddening in leaves due to lack of chlorophyll. If the
plant is experiencing high phosphorus deficiencies the leaves may become
denatured and show signs of death. Occasionally the leaves may appear purple
from an accumulation of anthocyanin because phosphorus is a mobile nutrient,
older leaves will show the first signs of deficiency. In plants, sulphur cannot
be mobilized from older leaves for new growth, so deficiency symptoms are seen
in the youngest tissues first. Symptoms of deficiency include yellowing of
leaves and stunted growth. The lowest content for nitrate, sulphate and
phosphorus is the soil from mountain. This explains no growth in the soil.
7.0
Conclusion
In conclusion, identification
of macronutrient was conclude in Table 5.1. From that table, soil sample of
lake of Residential College E recorded the highest macronutrient than other
soil sample. So this is suitable for jagung
pandan growth. Nutrient is very important for jagung pandan growth. Based on our observation, the leaf of jagung pandan is yellowish and purplish
in colour. This indicates our jagung
pandan is lack of phosphorus. But we cannot add on any fertilisers because
we want to measure the origin nutrient inside each of our sample soil.
8.0 References







Comments
Post a Comment